Stoichiometry comes from the Greek
word stoicheion which means element and metron which means measure.
Stoichiometry discusses the relation of mass between elements in a compound
(stoichiometry compound) and interactivity in a reaction (reaction stoichiometry).
Mass measurements in chemical reactions were initiated by Antoine Laurent
Lavoisier (1743 - 1794) who found that in chemical reactions there was no mass
change (mass conservation law). Furthermore Joseph Louis Proust (1754 - 1826)
found that the elements form compounds in certain comparisons (fixed comparison
law). Furthermore, in order to construct his atomic theory, John Dalton
discovered the third basic chemical law, called the law of multiples of
comparison. These three laws are the basis of the first chemical theory, the
atomic theory proposed by John Dalton around 1803. According to Dalton, every
material consists of atoms, elements composed of similar atoms, whereas
compounds composed of different atoms in certain comparisons .
However, Dalton has not been able to
determine the ratio of the atoms in the compound (the chemical formula of the
substance). Determination of chemical formula of substances can be done thanks
to the discovery of Gay Lussac and Avogadro. Once the chemical formula of the compound
can be determined, then the ratio of Inter atom (Ar) and inter molecular (Mr)
masses can be determined. Knowledge of relative atomic mass and chemical
formula of compounds is the basis of chemical calculations.
A. Atomic Mass
1. Average atomic mass
Atoms of the same element do not always have the same mass. This
is known as isotopes. The atoms in nature can have different masses, then the
atomic mass is calculated on the average mass of all the atoms in nature.
Example:
The chlorine atoms in nature are present in two isotopes, 75% as
Cl-35 with mass 35 s.m.a, and 25% as Cl-37 with a mass of 37 s.m.a. Then the
average mass of chlorine atoms is:
2.
relative atomic mass (Ar)
Measuring
the mass is comparing the mass of an object to another, in which the mass of
the reference object is called the standard mass.
Example:
If
the mass of 1 atom C-12 is 2.04 x 10-27 kg. What is the average mass of 1
magnesium atom, if Ar Mg = 24?
Answer:
An
average mass of 1 atom Mg = 4.08 x 10-27kg
3.
Relative Molecular Mass and Mass Relative Formula (Mr)
The mass of a molecule of a compound is called the relative molecular mass (Mr). The magnitude of the relative molecular mass of a compound is the sum of the relative atomic mass (Ar) of its constituent elements.
Mr
AxBy = (x Ar
A + y Ar B)
example
Calculate
the relative molecular mass of H2O
Mr=
(2 × atomic mass of hydrogen) + (1 × mass of oxygen atom)
Mr=
(2 × 1) + (1 × 16) = 18.
The
multiplier number in front of the atomic mass of each element is the index of
the element in the compound.
B. Mol
For practical reasons, in determining
the particle size, chemists agree to look for units called moles. One mole is a
number of particles contained in a substance equal to the number of atoms
present in 12.00 grams of C-12.
From the experiments performed by
Joseph Loschmidt and then justified by Avogadro, it turns out that the number
of carbon atoms contained in 12.00 grams of C-12 is 6.02 x 1023butir atom. This
number is hereinafter called Avogadro number or Avogadro constant and given the
symbol L.
1 mol of substance = 6.02 x 1023particles
1. Molar Mass
The mass relationship with the number
of particles is expressed in the molar mass. The molar mass is the mass of the
substance that is equal to the atomic mass or the mass of the formula of the
substance expressed in grams.
Molar mass (M) = Mass 1 mol of substance X = (ArX) gram
Since Mr of a molecule or unit of chemical formula of a compound
is the amount of Ar of its constituent atoms, then:
Molar mass (M) = Mass 1 mol of substance AxBy = (MrAxBy) gram
By means of the molar mass (M), the number of moles of a
substance can be calculated by:
With n = the number of moles of the substance
A = the mass of matter in a gram
M = molar mass = Mr in grams
Example:
Calculate how many mol molecules are contained in 6gram glucose
(C6H12O6) if known Ar C = 12, O = 16, and H =
1.
Answer:
2. Molar Volume
The molar volume of the gas is the volume of 1
mole of gas at a given temperature and pressure. To determine the molar volume
of the gas in the standard state we are weighing a certain amount of gas volume
in the already known empty mass tube at 00 and the gas pressure of 1
atm. The standard state (00C, 1 atm) volume of 1 mol of gas is 22.4
liters.
V = n mol x 22.4 L / mol
With V = gas volume at 00C, 1 atm
n = number of moles of gas
Example:
Calculate the volume of 4 grams of SO3 gas if it is
known Ar S = 32, O = 16.
Answer:
C.
The basic laws of chemistry
The
basic laws of chemistry are:
1.
Law eternity mass
2.
Law comparison permanent
3.
Dalton's law or law comparison Multiple
4.
Gay-Lussac Law or law Volume comparison
5.
Avogadro's law





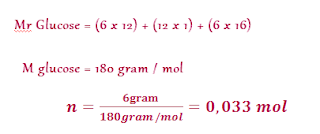

What is stoichiometric difference with the concept of mole?
BalasHapusStoichiometry is a science that studies the quantitative aspects of chemical reactions or chemical formulas. The quantitative aspect is obtained by measuring the mass, volume, number and so on, which are related to the number of atoms, ions, molecules or chemical formulas, and their correspondence in a chemical reaction.
HapusWhile the concept of moles is used To simplify the number of particles that are unusually small. Mol represents the unit of quantity of matter. Unit amount of this substance as well as the simplification of the amount of a good.
"The law of conservation of the masses", what does it say and give an example?
BalasHapusThe Law of Conservation of Mass (Lavoisier Law)
Hapus"The mass of the substance before the reaction equals the mass of the substance after the reaction"
The example of mass conservation law is as follows: If we mix or react sulfur with mass of 32 g and copper with a mass of 63.5 g then produce copper (II) sulphide with mass = mass of sulfur + copper mass (32 g + 63.5 g = 95.5 g). However, in some cases such as burning paper and has become ash. It may be that the ash is lighter than paper so the reaction is due to other reactions such as ash and CO2 gas lost in the wind.
give me another example about how to find Relative Molecular Mass and Mass Relative Formula (Mr) ?
BalasHapusExample, what is the relative molecular mass of the kitchen salt (NaCl) and sulfuric acid (H2SO4) if known relative atomic mass Na = 23, Cl = 35, H = 1, S = 32, and O = 16?
HapusAnswer:
Relative Molecular Mass NaCl = Ar Na + Ar Cl = 23 + 35 = 58 sma
H2SO4 = 2 Ar H + Ar S + 4 Ar O = 2 (1) + (32) + 4 (16) = 2 + 32 + 64 = 98 sma
3. Hydrogen gas can be made from the reaction of Zinc metal with sulfuric acid solution. Calculate the 2 M sulfuric acid volume required to produce 6.72 liters of hydrogen gas (STP)?
BalasHapusZn + H2SO4 => ZnSO4 + H2
Hapus2M 6,72 L
nH2 = VH2/VSTP
= 6,72L/22,4 L
= 0,3 mol
nH2SO4=(koefisien H2SO4)/(koefisien H2)×nH2
nH2SO4=1/1×0,3mol
=0,3mol
n = M x V
V=n/M
V = 0,3 mol/2
V= 0,15 L
What to consider in molar mass calculations?
BalasHapusWhich needs to be considered for finding the molar mass that is the relative atomic mass and also the coefficient or the number of elements or particles present in a compound then multiplied by the relative atomic mass. The unit of the molar mass is grams / mol. Of this molar mass, we can find the number of moles of a substance.
HapusWhat are some of the basic chemical laws you have mentioned?
BalasHapus1. The Law of Conservation of Mass (Lavoisier Law)
Hapus"The mass of the substance before the reaction equals the mass of the substance after the reaction"
2. Comparable Law (Proust Law)
"The mass ratio of the constituent elements is always fixed, even if it is made in a different way"
3. Dalton's law or law comparison Multiple
"When two elements react to form two or more compounds, the weight ratio of one element reacting with a certain weight of the other element in both compounds is always a simple integer ratio."
4. Comparative Law of Volume (Gay Lussac Law)
Applies only to chemical reactions that involve the gas phase
"At the same temperature and pressure, the ratio of reactant gas volume to the gas volume of the reaction product is a simple integer (equal to the ratio of the reaction coefficient)"
5. Avogadro's Law
Applies only to chemical reactions that involve the gas phase
"At the same temperature and pressure, the same volumes of gases contain the same number of moles"
Can we change the number of gas constant? Why?
BalasHapusWe can not change the gas contours because the gas constants are determined exactly. The gas constants (also called ideal, molar, universal, or universal gas constants, usually denoted by the letter R) are a physical constant that often appears in many fundamental physical equations, such as the ideal gas law and Nernst's equation. This constant is equivalent to the Boltzmann constant, but expressed in units of energy per kelvin per mole (rather than energy per kelvin per particle).
HapusThe price is:
R = 8.314472 (15) J · K-1 · mol-1
The two digits inside the brackets are the uncertainty (standard deviation) at the last two digits.
What is relationship between mole and volume?
BalasHapusThe mole and volume relationships are divided into two in standard and non-standard state.
Hapus1.Gas on the standard state
Measurement of gas quantity depends on temperature and gas pressure. If the gas is measured in a standard state, then the volume is called the molar volume. The molar volume is the volume of 1 mole of gas measured under standard circumstances. Standard circumstances are conditions at 0 ° C (or 273 K) and atmospheric pressure (or 76 cmHg or 760 mmHg) or STP (Standard Temperature and Pressure).
The amount of gas molar volume can be determined by the ideal gas equation: PV = nRT
P = pressure = 1 atm
N = mol = 1 mole of gas
T = temperature in Kelvin = 273 K
R = gas constant = 0.082 liter atm / mol K
Then:
P V = nRT
V = 1 x 0.082 x 273
V = 22,389
V = 22.4 liters
Thus, the standard volume = VSTP = 22.4 Liter / mol.
Can be formulated: V = n x Vm
N = number of moles
Vm = VSTP = molar volume
Problems example:
1) What quantity (in moles) of hydrogen gas is 6.72 liters, when measured at 0 ° C and 1 atm pressure?
Answer:
Quantity (in moles) H2 = volume H2 / VSTP
= 6.72 L / 22.4 mol / L
= 0.3 mol
2. Gas in a non-standard state
If the gas volume is measured on the ATP (Am-bient Temperature and Pressure) or better known as non-STP then use the formula:
P V = n R T
P = pressure, the unit P is the atmosphere (atm)
V = volume, unit V is liter
N = mol, the unit n is the mole
R = gas constant = 0.082 liter atm / mol K
T = temperature, unit T is Kelvin (K)
Problems example:
Determine the volume of 1.7 grams of ammonia gas measured at a temperature of 27 ° C and a pressure of 76 cmHg!
Answer:
N = mass of ammonia / molar mass of ammonia
= 1.7 grams / 17 grams / mol
= 0.1 mol
P = (76 cmHg / 76 cmHg) x 1 atm = 1 atm
T = (t + 273) K = 27 + 273 = 300 K
P V = n R T
1 atm × V = 0.1 mol × 0.082 L atm / mol K × 300 K
V = 2.46 L
what do you mean by stoichiometry ?
BalasHapusStoichiometry is a chemical branch associated with a quantitative relationship that exists between reactants and products in chemical reactions. The reactant is a substance that participates in a chemical reaction, and also the product is a substance obtained as a result of a chemical reaction.
HapusWhat is the avogadro number ?
BalasHapusThe avogadro number is the number of molecules present in a mole or the weight of a gram of molecule of any material. One gram of molecular weight is the weight of a substance (in grams) that is numerically equivalent to the dimensionless molecular weight of the substance. The number of molecules in a gram of molecular weight has been determined approximately about 6.0221367x10^23 molecules.
HapusSpecify some isotopes of some elements in the periodic table?
BalasHapusIsotopes are elements that have the same number of Atoms (Protons) but their mass numbers (Neutrons) are different. Since the atomic number is the identity of an element, then the isotope, even though it has a different mass number, remains in one element. Hence in the periodic table, the entire Isotope of an element lies in the same place. The isotopes of each element will have the same number of valence electrons that will inevitably have the same chemical properties.
HapusExamples of Isotopes include:
Hydrogen (H) 1H1, 1H2, 1H3
Helium (He) 2He3, 2He4,
Carbon (C) 6C12, 6C13, 6C14
Nitrogen (N) 7N14, 7N15
Oxygen (O) 8O16, 8O17, 8O18
Description: the atomic number (proton) to the left of the element, the mass number (neutron) to the right of the element.
The isotope of an element though has almost the same chemical properties, but its characteristics and physical properties differ between isotopes of one with another isotope in one element. This is because the physical properties of the elements are usually influenced by the number of Neutrons present in the Nuclei. The physical properties of elements such as melting point, boiling point, density and others depend on the atomic period. Since the number of times an Isotope is not the same, then the atomic mass automatically is different.
What are the counts included in stoichiometry?
BalasHapusWhich are included in the stoichiometric discussion are as follows:
Hapus-Mon Concept
-Meam Measurement of Atom-Atoms
-Molor Measures of Compounds
-Preposition Percent
- Empirical Formulas and Molecules
Chemical Reactions and Reaction Equations
-Counts Based on Reaction Equations
-Re calculation of Limiting Reagents
-Molar Concentration
-Dilution
-Stoichiometry Reaction in Solution
-Stoichiometry Gas Phase Reaction
Explain the comparison of permanent law
BalasHapusThe law of fixed comparison or commonly referred to as Proust Law is a law which states that all compounds comprise a constant mass constituent of constituent elements.
HapusThis law was put forward by Joseph Lonis Prost (1754-1826) originating from France. The fixed ratio law reads: "The weight ratio of the compounding elements of the compound is fixed". Experiments doneProust is a reaction between the elements of hydrogen and oxygen to form water (H2O).